DNA REPAIR MECHANISMS IN PLASMODIUM:
Our research aims at understanding the biology of malaria parasites with a goal of identifying novel drug targets. Our work has identified the homologous recombination (HR) pathway of the parasite as an excellent target. Unrepaired DNA double strand breaks (DSB) are lethal for the parasites. We have shown that in Plasmodium such DSBs are repaired only by HR pathway. We have observed that other DSB repair mechanisms operational in other eukaryotes, namely the non-homologous end joining (NHEJ) mechanism, is not functional in this parasite (Roy et al. Mol. Micro. 2014). The significance of this finding is that one can target the HR pathway of the parasite since there is no compensatory mechanism. Our group has developed mutant parasite that is incapable of performing HR. Mice infected with such parasites live significantly longer with reduced parasite burden (Roy et al. Mol. Micro., 2014). Thus, our work implies that the HR pathway of Plasmodium could be efficiently targeted to curb malaria. To that end our lab has identified the sensor of DSB (PfalMre11); the strand-invasion recombinase PfRad51; and the resolvase PfBlm, and established the essential roles of these proteins in Plasmodium biology. We have discovered two small molecule inhibitors targeting PfRad51 or PfBlm with significant anti-parasitic activity. More importantly, these two HR inhibitors work synergistically with two first-line anti-malarial drugs, Artemisinin (ART) and Chloroquine (CQ), as both ART and CQ create genome-wide DSBs in Plasmodium (Vydyam et al. JBC, 2019; Suthram et al. mSphere, 2020) (Figure 1).

In addition to identifying novel drug targets, our work has unravelled several biological mysteries pertaining to the DSB repair mechanisms in the apicomplexan parasites. For example, between the two sister apicomplexan parasites Toxoplasma predominantly utilizes the NHEJ pathway, while Plasmodium uses only HR pathway. Our work has suggested that a weaker ATP hydrolysis activity of TgRad51 could be responsible for the choice against HR (Achanta et al. PLoSONE, 2012). In other eukaryotes Mre11 plays a deciding role while choosing between HR and NHEJ. We have demonstrated that PfalMre11 possesses only a sub-set of Mre11 activities (Badugu et al. PLoSONE, 2015). However, heterologous expression of PfalMre11 in an organism equipped with both HR and NHEJ, PfalMre11 could carry out NHEJ. Thus, our finding suggests that the lack of other proteins of the NHEJ pathway such as Ku70/80 could be responsible for a non-functional NHEJ in Plasmodium (Badugu et al. PLoSONE, 2015).
We have recently discovered that PfRad51 is a client of PfHsp90 (Tabassum et al. Antimicrobial Agents and Chemotherapy, 2021). This has a huge implication in parasite biology as the parasites are not only exposed to various temperature shocks during its propagation within the human host but also experience deleterious DSB from both exogenous and endogenous sources. Thus, unravelling the connection between DNA repair protein Rad51 and Hsp90 opens up several avenues for future research. Our discovery that the DNA damage resolvase PfBlm is also regulated by PfHsp90 highlighted a central regulatory role of Hsp90 in DSB repair.
GENETIC AND EPIGENETIC REGULATIONS OF ANTIGENIC VARIATIONS:
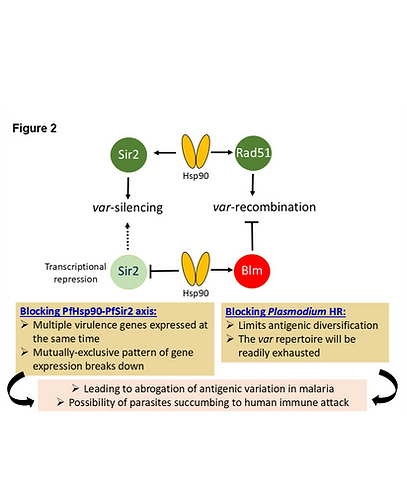
Another interest of our lab is to unravel the molecular mechanism of antigenic variation in P. falciparum. As antigenic variation is a potent immune evasion mechanism, it is of utmost important to decipher the molecular mechanism of this phenomenon. In a collaborative work we have uncovered the involvements of PfHsp90 in the trafficking of these variable antigens on RBC surface (Jha et al. MBP, 2017). It has been implicated that the histone deacetylase PfSir2 plays a pivotal role in regulating mutually exclusive expression of the variable antigens. In other eukaryotes, Sir3 plays a key role in recruiting the Sir2 complex at the sub-telomeric regions. Interestingly, Plasmodium lacks Sir3. We have discovered that one of the replication proteins, PfOrc1 performs a Sir3 like function in Plasmodium (Varunan et al. MBP, 2013). In a recent work we have uncovered that exposure to febrile temperature modulates the expression of virulence genes that could impact chronicity of malaria infection. Our group found that the master epigenetic regulator PfSir2 is itself regulated transcriptionally by epigenetic modification in a Hsp90 dependent manner (Tabassum et al. Molecular Microbiology, 2021). The over-production of Hsp90 due to fever, results in lowering of the PfSir2 regulator. This leads to the simultaneous expression of multiple virulence genes, hence the antigenic variation in the parasite becomes dysregulated (Tabassum et al. Mol. Micro., 2021). Further we have uncovered that PfHsp90 not only regulate the transcription of PfSir2, it also provides functional maturation to PfSir2 through its canonical chaperoning function (Tabassum et al. mSphere, 2022). In another work we demonstrated that the Hsp90 client PfRad51 is instrumental for the diversification of the variable antigens in this parasite (Vydyam et al, Front. Bio Science, 2023). Thus, Hsp90 regulates both the arm of antigenic variation: antigenic diversification and antigenic switching (Figure 2).
Currently we are investigating how the crosstalk between the HR and MMR (Mismatch Repair) pathways maintains a delicate balance between genomic integrity and genomic plasticity in this parasite. This involves the identification and functional characterization of newer players from both the DNA repair pathways.